玻璃酸钠在骨科和运动医学相关疾病中的应用专家共识(2017年修订版)
2017-11-14 文章来源:中国医师协会骨科医师分会运动医学专业委员会 点击量:9482 我要说
1 背景
为规范玻璃酸钠(hyaluronan,HA)在骨科和运动医学相关疾病治疗中的合理应用,为临床医生提供较为详实、准确、实用的HA临床应用指导意见,人民卫生出版社《中国医学前沿杂志(电子版)》编辑部曾先后于2010年1月9日和2012年9月16日组织并邀请了全国数十位知名骨科和运动医学专家在北京召开了“玻璃酸钠在骨科中的应用专家研讨会”[1]和“玻璃酸钠在骨关节炎治疗中的应用专家共识研讨会”,并最终达成共识。《玻璃酸钠在骨关节炎治疗中的应用专家共识(2012年版)》[2](以下简称为2012版共识)全文发表于《中国医学前沿杂志(电子版)》2012年第11期。
2012版共识自2012年11月发表以来已历时5年,深受广大骨科和运动医学科医师的好评,HA的临床应用已被收录入国家卫生计生委发布的《骨关节炎临床路径》(2016年版),进一步确立了HA在骨关节炎(osteoarthritis,OA)治疗中的重要地位;同时,结合近年来2012版共识在骨科和运动医学领域的应用情况以及循证医学证据和临床实践经验的不断积累,由中国医师协会骨科医师分会运动医学专业委员会和人民卫生出版社《中国医学前沿杂志(电子版)》编辑部联合发起的《玻璃酸钠在骨科和运动医学相关疾病中的应用专家共识》修订工作应运而生。
2017年9月23日,“玻璃酸钠在骨科和运动医学相关疾病中的应用专家共识修订研讨会”在宁波召开,来自全国的20余位骨科和运动医学领域知名专家参加了研讨会,并就修订内容进行深入讨论。专家们本着严谨、客观、公正的科学精神,经过反复推敲、论证,数易其稿,历时2个月,最终定稿。修订版共识旨在使更多骨科、运动医学科医师和相关疾病患者获益。
2 玻璃酸钠概述
1934年,Meyer等[3]发现HA广泛分布于人和动物各组织的细胞外基质(如玻璃体、关节滑液、滑膜、软骨等),是一种高分子量多糖,相对分子量为20万~720万Da。HA包含内源性(即人体自身分泌的)和外源性(即外来补充的)两种。当内源性HA的产生和代谢出现异常,并导致组织、器官的生物学功能障碍,出现临床症状时,可通过补充外源性HA达到治疗效果。外源性HA的制备来源主要为发酵、提取及化学合成(交联),哪种工艺更好,目前尚无定论。目前的研究表明:总体来看,上述来源的HA均安全、有效。
2.1 作用机制 临床研究表明:HA对于关节疼痛有长期疗效,HA注射后5~13周,患者疼痛改善11%~54%。但其具体作用机制仍存在一些争议,总结文献,其主要作用机制如下:
(1)保护软骨细胞:通过与HA受体细胞黏附分子(cluster of differentiation 44,CD44)和透明质酸调节的运动受体(hyaluronan-mediated motility receptor,RHAMM)结合,减少白介素(interleukin,IL)-1β、前列腺素E2、基质金属蛋白酶(matrix metalloproteinase,MMP)-1、MMP-2、MMP-3、MMP-9、MMP-13及自由基的合成和释放,降低炎性细胞的数量,减少软骨细胞凋亡,促进软骨细胞增殖[4-6]。
(2)促进蛋白聚糖和糖胺聚糖合成:外源性HA通过CD44和细胞间黏附分子1(intercelluar adhesion molecule-1,ICAM-1)结合的影响,促进软骨细胞合成及蛋白聚糖和糖胺聚糖分泌[7-10]。
(3)抗炎:通过与HA受体CD44和RHAMM结合,减少肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、IL-1β、IL-6、IL-17、MMP-13及诱导型一氧化氮合酶(inducible nitric oxide synthase,iNOS)的合成[11-14]。
(4)机械润滑:外源性HA增加滑液非牛顿流体的特性和黏弹性。当关节处于低撞击频率时(如正常行走时),HA发挥润滑功能,减少组织间的摩擦;当关节处于高撞击频率或负重时,滑液由黏性特征转换为弹性特征,缓冲应力对关节的撞击,对关节软骨等发挥保护作用[15-17]。
(5)保护软骨下骨:通过与HA受体CD44结合,抑制MMP-13和IL-6[6,18-21]。
(6)镇痛:通过降低关节内机械感受器的牵张机械感度、减少致痛性神经肽的分泌,达到镇痛效果[22-24]。
(7)促进内源性HA分泌:通过促进内源性HA合成来改善病理性关节液的性状,持续缓解症状,延缓病情进展[25-29]。
(8)保护半月板:通过润滑发挥保护半月板的作用[30]。
2.2 药物代谢 大多数HA产品的半衰期不超过1天。注入关节腔的HA,2小时可渗入至滑膜组织、韧带、相邻肌肉组织及肌间隙,6小时可进入软骨组织,3~8小时在血浆中可检测到,72小时在关节腔的残留量仅为投药量的10%[31,32]。通过多种方法可将HA分子连接在一起,产生交联HA,其在关节内具有较长的半衰期,可达1.5~9天[33-35]。
关节组织内浓度:在关节液中几乎不代谢而渗入滑膜组织。高浓度分布于滑膜和韧带组织内,其次为半月板和关节软骨。
代谢:降解呈低分子化后进入血液,主要在肝脏被代谢[36]。
排泄:代谢产物大部分以二氧化碳形式经呼吸运动排出,一部分经尿和粪便排泄。
2.3 安全性及不良反应 HA生物相容性良好,能在体内完全代谢,无毒、无菌、无趋化作用,不引起异物反应,不与细胞和蛋白相互作用,因而总体安全性良好[37]。
HA的常见不良反应主要为注射局部和关节出现轻微或中度疼痛和肿胀积液,发生率为1%~15%,偶有头痛、发热及药疹。上述不良反应多于注射后24小时内发生,患者一般能耐受,无需特殊处理,2~3天后症状消失。注射技巧、注射后患者活动以及产品纯度等均为影响局部不良反应发生的因素[38-51]。感染并非HA的不良反应,而是由于注射全程无菌操作技术不当所致。研究表明,生物发酵来源的HA产品与禽类提取来源的HA产品因治疗相关不良事件导致的停药率相似[45,49-51]。
2.4 目前国内市场部分已有HA产品(表1,以首字母排序)
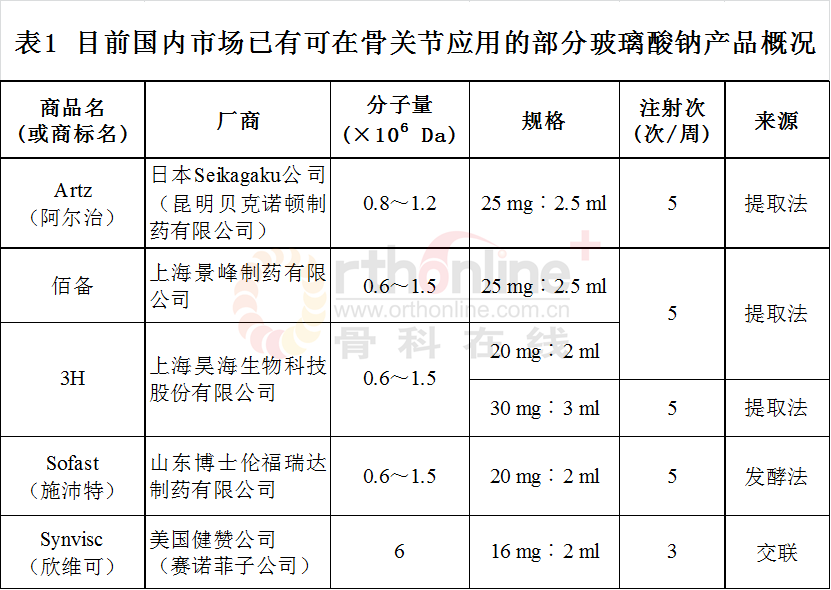
3 玻璃酸钠在骨科和运动医学相关疾病中的应用
根据目前国内外文献报道,HA可用于多关节OA、运动损伤性疾病及关节镜术后,对于缓解患者疼痛、改善关节功能等具有良好疗效,特别适用于非甾体抗炎药(non-steroid anti-infl ammatory drugs,NSAIDs)和镇痛药疗效欠佳,或无法使用上述药物的患者。有证据表明,HA治疗早、中期OA具有较好的卫生经济成本效益,可减少NSAIDs等镇痛药用量[52-56]。通过对文献中HA在骨科和运动医学相关疾病中的应用及疗效分析,总结适应证、禁忌证及用法等如下。
3.1 适应证和禁忌证
3.1.1 适应证
(1)大关节OA非急性肿胀期,常用于膝、肩、踝、髋、肘、腕等关节,尤其是轻、中度OA(Kellgren-Lawrence Ⅰ~Ⅲ级),对于缓解疼痛、改善关节功能等具有良好疗效,且安全、耐受[46,57-75]。
(2)用于NSAIDs等镇痛药禁忌或无效的患者,可缓解疼痛、改善关节功能[46,54,75,76]。
(3)用于不适合全膝关节置换(total knee arthroplasty,TKA)手术或希望延缓手术时间的重度膝OA(Kellgren-Lawrence Ⅳ级)患者[75,77-81]。
(4)用于上述关节OA关节镜清理术后,可缓解术后关节疼痛、改善关节功能[82-91]。
(5)用于关节镜下半月板损伤修整成形术后,可缓解术后短期膝关节疼痛、改善关节功能,减少NSAIDs用量[84,92-94]。
(6)用于冻结肩、肩峰下撞击综合征、肩袖部分损伤等疾病及关节镜术后,能缓解疼痛、改善关节功能[95-101]。
(7)用于股骨髋臼撞击综合征,能缓解疼痛、改善关节功能,但并不能延缓疾病进程[102-107]。
(8)用于踝关节距骨骨软骨损伤术后,能缓解疼痛、改善关节功能、促进微骨折术后纤维软骨再生[88,108]。
(9)用于肌腱病,如冈上肌腱腱病、跟腱腱病及扳机指等,可预防粘连、减轻疼痛、改善关节功能[109-113]。
3.1.2 禁忌证
(1)关节内感染,关节穿刺局部皮肤破溃感染。
(2)凝血功能异常。
(3)对禽类和蛋类过敏患者应慎用。
(4)不能排除其他疾病引起的关节明显肿胀和积液。
3.2 用法及用量(具体参照不同产品的使用说明执行)
(1)用法:①对于关节内疾病,HA的使用方法为关节腔内注射,不能注入软组织内。严格遵循无菌操作规范和正确的关节穿刺技术,保证药物注射入关节腔尤为重要。避免反复穿刺损伤软组织及关节软骨;注射前有关节积液时应先将其抽除。②对于肌腱病,HA应准确注射于病变肌腱的腱鞘或滑囊内。
(2)用量:每个关节每次注射剂量为1支单位,每周注射1次;根据药物不同,3~5周为1个疗程。
(3)重复治疗:患者接受HA治疗应根据病情进展而定,如病情需要仍适合HA治疗,一般可6~12个月后重复治疗[40,114]。
(4)HA在急性炎症缓解、手术创伤反应及肿胀减轻后用于关节内注射效果更佳[73,80-83]。
(5)HA最好不与糖皮质激素及局部麻醉药在体外混合后一起注射[115],但可分管先后注射,联合使用。
3.3 不良事件处理
(1)注射局部及关节腔反应:表现为注射局部轻-中度疼痛、肿胀或关节内少量积液,一般多能耐受,无需特殊治疗,也可采取休息、冰敷或使用NSAIDs等处理措施。一般2~3天后症状改善并恢复[40-47,51]。
(2)过敏反应:很少见,主要表现为荨麻疹、恶心、呕吐、发热、水肿(颜面、眼睑等)、颜面发红等,偶见过敏性休克[40,45,51]。如发现过敏反应,立即停药,并作相应抗过敏处理。对禽类和蛋类过敏患者应慎用HA。
(3)注射后关节化脓性感染:少见,一般可能因医务人员消毒不严格引起,应注意与注射后关节腔一过性积液鉴别,如确诊为关节感染则应按感染性关节炎治疗[40,45,51,57]。
4 结束语
HA可长期缓解疼痛、维护并改善关节功能,其不良反应轻微且发生率低。另外,HA可减少NSAIDs等口服镇痛药的用量,特别适用于老年、既往有消化道溃疡病史、出血史、心脑血管疾病病史的患者,可减少其他药物带来的胃肠道不良反应及心血管不良事件。HA可用于治疗膝、肩、踝、髋、肘、腕等关节的OA,以及上述关节和肌腱的运动损伤、退变性疾病,也可用于关节镜术后作为黏弹性药物注射。HA的使用应在准确诊断的基础上,严格把握其适应证,掌握正确的使用方法和剂量,以及联合用药,以达到最佳的临床效果。
《玻璃酸钠在骨科和运动医学相关疾病中的应用专家共识(2017年修订版)》参考了国际、国内最新文献,同时结合国内实际情况,并结合HA使用的安全性、有效性、实用性及经济性,为我国临床医师在骨科和运动医学领域充分、规范地使用HA提供参考意见。
会议主席:
陈世益(复旦大学附属华山医院)
王坤正(西安交通大学第二附属医院)
专家共识委员会名单:(按姓氏拼音排序)
白伦浩 毕 擎 陈世益 戴国锋
黄竞敏 李 箭 李 棋 李国平
李宏云 李卫平 林 朋 刘 宁
刘玉杰 吕 伟 吕红斌 孙 康
王 飞 王 洪 王 蕾 王 青
王坤正 王满宜 卫小春 伍 骥
邢更彦 徐卫东 薛庆云 杨 柳
查振刚 张 磊 张晓南 郑 江
赵金忠
执笔专家:
李 箭(四川大学华西医院)
李 棋(四川大学华西医院)
李宏云(复旦大学附属华山医院)
陈世益(复旦大学附属华山医院)
利益冲突声明:本共识的修订过程中,赛诺菲(北京)制药有限公司、上海昊海生物科技股份有限公司、山东博士伦福瑞达制药有限公司、昆明贝克诺顿制药有限公司赞助了修订会议。
参考文献:
[1] 李箭,裴福兴.玻璃酸钠在骨科中的应用专家研讨会会议纪要[J].中国医学前沿杂志(电子版),2010,2(1):75-80.
[2] 玻璃酸钠在骨关节炎治疗中的应用专家委员会.玻璃酸钠在骨关节炎治疗中的应用专家共识(2012年版)[J].中国医学前沿杂志(电子版),2012,4(11):1-7.
[3] Meyer K, Palmer JW. The polysaccharide of the vitreoushumor[J]. J Boil Chem, 1934, 107(3):629-634.
[4] Chang CC, Hsieh MS, Liao ST, et al. Hyaluronan regulates PPARgamma and inflammatory responses in IL-1betastimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym, 2012, 90(2):1168-1175.
[5] Altman R, Manjoo A, Fierlinger A, et al. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review[J]. BMC Musculoskeletal Disorders, 2015, 16:321.
[6] Hiraoka N, Takahashi Y, Arai K, et al. Hyaluronan and intermittent hydrostatic pressure synergistically suppressed MMP-13 and Il-6 expressions in osteoblasts from OA subchondral bone[J]. Osteoarthr Cartil, 2009, 17(1):S97.
[7] Maneiro E, de Andres MC, Fernandez-Sueiro JL, et al. The biological action of hyaluronan on human osteoartritic articular chondrocytes: the importance of molecular weight[J]. Clin Exp Rheumatol, 2004, 22(3):307-312.
[8] Miki Y, Teramura T, Tomiyama T, et al. Hyaluronan reversed proteoglycan synthesis inhibited by mechanical stress: possible involvement of antioxidant effect[J]. Inflamm Res, 2010, 59(6):471-477.
[9] Yatabe T, Mochizuki S, Takizawa M, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes[J]. Ann Rheum Dis, 2009, 68(6):1051-1058.
[10] Williams J. The effects of hyaluronic acid on fibronectin fragment mediated cartilage chondrolysis in skeletally mature rabbits[J]. Osteoarthr Cartil, 2003, 11(1):44-49.
[11] Galois L, Etienne S, Henrionnet C, et al. Ambivalent properties of hyaluronate and hylan during post-traumatic OA in the rat knee[J]. Biomed Mater Eng, 2012, 22(4):235-342.
[12] Smith MM, Russell AK, Schiavinato A, et al. A hexadecylamide derivative of hyaluronan (HYMOVIS®) has superior beneficial effects on human osteoarthritic chondrocytes and synoviocytes than unmodified hyaluronan[J]. J Inflamm (Lond), 2013, 10:26.
[13] Oliviero F, Scanu A, Ramonda R, et al. Mechanisms involved in inhibition of inflammation in THP-1 cells by the hexadecylamide derivative of hyaluronic acid[J]. Osteoarthr Cartil, 2014, 22:S292-S293.
[14] Campo GM, Avenoso A, Nastasi G, et al. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression[J]. Biochim Biophys Acta, 2011, 1812(9):1170-11781.
[15] Lu HT, Sheu MT, Lin YF, et al. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis[J]. BMC Vet Res, 2013, 9:68.
[16] Plaas A, Li J, Riesco J, et al. Intraarticular injection of hyaluronan prevents cartilage erosion, periarticular fibrosis and mechanical allodynia and normalizes stance time in murine knee osteoarthritis[J]. Arthritis Res Ther, 2011, 13(2):R46.
[17] Waller KA, Zhang LX, Fleming BC, et al. Preventing friction-induced chondrocyte apoptosis: comparison of human synovial fluid and Hylan G-F20[J]. J Rheumatol, 2012, 39(7):1473-1480.
[18] Ding M, Christian Danielsen C, Hvid I. Effects of hyaluronan on three-dimensional microarchitecture of subchondral bone tissues in guinea pig primary osteoarthrosis[J]. Bone, 2005, 36(3):489-501.
[19] Armstrong S, Read R, Ghosh P. The effects of intraarticular hyaluronan on cartilage and subchondral bone changes in an ovine model of early osteoarthritis[J]. J Rheumatol, 1994, 21(4):680-688.
[20] Hiraoka N, Takahashi KA, Arai Y, et al. Intraarticular injection of hyaluronan restores the aberrant expression of matrix metalloproteinase-13 in osteoarthritic subchondral bone[J]. J Orthop Res, 2011, 29(3):354-360.
[21] Prasadam I, Crawford R, Xiao Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes–possible pathogenic role in osteoarthritis[J]. J Rheumatol, 2012, 39(3):621-634.
[22] Yoshioka K, Yasuda Y, Kisukeda T, et al. Pharmacological effects of novel cross-linked hyaluronate, Gel-200, in experimental animal models of osteoarthritis and human cell lines[J]. Osteoarthritis Cartilage, 2014, 22(6):879-887.
[23] Boettger MK, Kummel D, Harrison A, et al. Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain[J]. Arthritis Res Ther, 2011, 13(4):R110.
[24] Pena Ede L, Sala S, Rovira JC, et al. Elastoviscous substances with analgesic effects on joint pain reduce stretch-activated ion channel activity in vitro[J]. Pain, 2002, 99(3):501-508.
[25] Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases[J]. Physiol Rev, 2011, 91(1):221-264.
[26] Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action[J]. Arthritis Res Ther, 2003, 5(2):54-67.
[27] Monfort MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment[J]. Rheumatol Int, 1987, 7(3):113-122.
[28] McKee CM, Penno MB, Cowman M, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages[J]. J Clin Invest, 1996, 98(10):2403-2413.
[29] Bagga H, Burkhardt D, Sambrook P, et al. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee[J]. J Rheumatol, 2006, 33(5):946-950.
[30] Levillain A, Magoariec H, Boulocher C, et al. Effects of a viscosupplementation therapy on rabbit menisci in an anterior cruciate ligament transection model of osteoarthritis[J]. J Biomech, 2017, 58:147-154.
[31] Hori K, Kikkawa M, Takaichi M, et al. Autoradiography of keen joint after intra-articular administration of 14Csodium hyaluronate(14C-SL-1010)in rabbits[J]. Jpn Pharmacol Ther, 1993, 21(supple 2):201-222.
[32] Obara T, Yamaguchi T, Moriya Y, et al. Tissue distribution of fluorescein-labeled sodium hyaluronate in experimentallyinduced osteoarthritis[J]. Jpn Pharmacol Ther, 1993, 21(supple 2): 193-200.
[33] Conrozier T. Is the addition of a polyol to hyaluronic acid a significant advance in the treatment of osteoarthritis?[J]. Curr Rheumatol Rev, 2017. [Epub ahead of print]
[34] Conrozier T, Mathieu P, Rinaudo M. Mannitol allows to preserve the elastoviscous properties of hyaluronic acid in an in vitro model of oxidative stress[J]. Drug Res (Stuttg), 2013, 63(9):445-449.
[35] Heisel J, Kipshoven C. Safety and efficacy findings from a non-interventional study of a new hyaluronic acid/sorbitol formulation (GO-ON® matrix) for intra-articular injection to relieve pain and disability in osteoarthritis patients[J]. Drug Res, 2013, 63:445-449.
[36] 凌沛学.透明质酸[M].北京:中国轻工业出版社,2007.
[37] 姜海,梁晓军.外源性玻璃酸钠治疗骨性关节炎机制研究进展[J].中国骨与关节损伤杂志,2001,16(6):475.
[38] Wenz W, Breusch SJ, Graf J, et al. Ultrastructural findings after intraarticular application of hyaluronan in a canine model of arthropathy[J]. J Orthop Res, 2000, 18(4):604-612.
[39] Yanaki T, Yamaguchi T. Temporary network formation of hyaluronate under a physiological condition. Molecular-weight dependence[J]. Biopolymers, 1990, 30(3-4):415-425.
[40] Jüni P, Reichenbach S, Trelle S, et al. Efficacy and Safety of Intraarticular Hylan or Hyaluronic Acids for Osteoarthritis of the Knee[J]. Arthritis Rheum, 2007, 56(11):3610-3619.
[41] Puttick MP, Wade JP, Chalmers A, et al. Acute local reactions after intraarticular hylan for osteoarthritis of the knee[J]. J Rheumatol, 1995, 22(7):1311-1314.
[42] Martens PB. Bilateral symmetric inflammatory reaction to hylan G-F 20 injection[J]. Arthritis Rheum, 2001, 44(4):978-979.
[43] Pullman-Mooar S, Mooar P, Sieck M, et al. Are there distinctive inflammatory flares after hylan g-f 20 intraarticular injections?[J]. J Rheumatol Dec, 2002, 29(12):2611-2614.
[44] Goldberg VM, Coutts RD. Pseudoseptic reactions to hylan viscosupplementation: diagnosis and treatment[J]. Clin Orthop Relat Res, 2004, (419):130-137.
[45] Altman RD, Bedi A, KarlssonJ, et al. Product Differences in Intra-articular Hyaluronic Acids for Osteoarthritis of the Knee[J]. Am J Sports Med, 2016, 44(8):2158-2165.
[46] Henrotin Y, Raman R, Richette P, et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis[J]. Semin Arthritis Rheum, 2015, 45(2):140-149.
[47] Hamburger MI, Lakhanpal S, Mooar PA, et al. Intra-articular hyaluronans: A review of product-specific safety profiles[J]. Semin Arthritis Rheum, 2003, 32(5):296-309.
[48] Bjordal JM, Ljunggren AE, Klovning A, et al. Non-steroidal anti-inflam- matory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials[J]. Br Med J, 2004, 329:1317-1322.
[49] Patel PB, Patel TK. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials[J]. Eur J Rheumatol, 2017, 4(1):11-18.
[50] Labianca R, Sarzi-Puttini P, Zuccaro SM, et al. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain[J]. Clin Drug Investig, 2012, 32 Suppl 1:53-63.
[51] O'Hanlon CE, Newberry SJ, Booth M, et al. Hyaluronic acid injection therapy for osteoarthritis of the knee: concordant efficacy and conflicting serious adverse events in two systematic reviews[J]. Systematic Reviews, 2016, 5:186.
[52] Hatoum HT, Fierlinger AL, Lin SJ, et al. Cost-effectiveness analysis of intra-articular injections of a high molecular weight bioengineered hyaluronic acid for the treatment of osteoarthritis knee pain[J]. J Med Econ, 2014, 17:326-337.
[53] Kahan A, Lleu PL, Salin L. Prospective randomized study comparing the medico-economic benefits of Hylan GF-20 vs conventional treatment in knee osteoarthritis[J]. Joint Bone Spine, 2003, 7:276-281.
[54] Torrance GW, Raynauld JP, Walker V, et al. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 2 of 2): economic results[J]. Osteoarthritis Cartilage, 2002, 10(7):518-527.
[55] Thomas T, Amouroux F, Vincent P. Intra articular hyaluronic acid in the management of knee osteoarthritis: Pharmaco-economic study from the perspective of the national health insurance system[J]. PLoS One, 2017, 12(3):e0173683.
[56] Miller LE, Sloniewsky MJ, Gibbons TE, et al. Long-term clinical benefit and cost-effectiveness of an 8-week multimodal knee osteoarthritis management program incorporating intra-articular sodium hyaluronate (Hyalgan®) injections[J]. J Pain Res, 2017, 10:1045-1054.
[57] Evanich JD, Evanich CJ, Wright MB, et al. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis[J]. Clin Orthop Relat Res, 2001, 390(9):173-181.
[58] Lomander LS, Dalen N, Englund G, et al. Intraarticular hyaluronan injections in the treatment of osteoarthritis of the knee: A randomized, double blind, placebo controlled multicenter trial[J]. Ann Rheum Dis, 1996, 55(7):424-431.
[59] 尚西亮,陈世益,李云霞.透明质酸在运动医学中的应用[J].上海医药,2012,33(15):12-15.
[60] 李棋,唐新,裴福兴,等.透明质酸在骨关节疾病中的应用[J].中国组织工程研究与临床康复,2010,14(47):8835-8839.
[61] Xing D, Wang B, Liu Q, et al. Intra-articular Hyaluronic Acid in Treating Knee Osteoarthritis: a PRISMA-Compliant Systematic Review of Overlapping Meta-analysis[J]. Sci Rep, 2016, 6:32790.
[62] Bannuru RR, Schmid CH, Kent DM, et al. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis: A Systematic Review and Network Meta-analysis[J]. Ann Intern Med, 2015, 162(1):46-54.
[63] Richette P, Chevalier X, Ea HK, et al. Hyaluronan for knee osteoarthritis: an updated meta-analysis of trials with low risk of bias[J]. RMD Open, 2015, 1(1):e000071.
[64] Campbell KA, Erickson BJ, Saltzman BM, et al. Is Local Viscosupplementation Injection Clinically Superior to Other Therapies in the Treatmentof Osteoarthritis of the Knee: A Systematic Review of Overlapping Meta-analyses[J]. Arthroscopy, 2015, 31(10):2036-2045.
[65] Ishijima M, Nakamura T, Shimizu K, et al. Intra-articular hyaluronic acid injection versus oral non-steroidal anti-inflammatory drug for the treatment of knee osteoarthritis: a multi-center, randomized, open-label, non-inferiority trial[J]. Arthritis Res Ther, 2014, 16(1):R18.
[66] Wang CT, Lin J, Chang CJ, et al. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a metaanalysis of randomized controlled trials[J]. J Bone Joint Surg Am, 2004, 86:538-545.
[67] Bellamy N, Campbell J, Robinson V, et al. Viscosupplementation for the treatment of osteoarthritis of the knee[J]. Cochrane Database Syst Rev, 2006, (2):CD005321.
[68] Lo GH, LaValley M, McAlindon T, et al. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis[J]. JAMA, 2003, 290(23):3115-3121.
[69] Conrozier T, Jerosch J, Beks P, et al. Prospective, multi-centre, randomised evaluation of the safety and efficacy of five dosing regimens of viscosupplementation with hylan G-F 20 in patients with symptomatic tibio-femoral osteoarthritis: a pilot study[J]. Arch Orthop Trauma Surg, 2009, 129(3):417-423.
[70] Pal S, Thuppal S, Reddy KJ, et al. Long-Term (1-Year) Safety and Efficacy of a Single 6-mL Injection of Hylan G-F 20 in Indian Patients with Symptomatic Knee Osteoarthritis[J]. Open Rheumatol J, 2014, 8:54-68.
[71] Wang Y, Hall S, Hanna F, et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial[J]. BMC Musculoskelet Disord, 2011, 12:195.
[72] Chareancholvanich K, Pornrattanamaneewong C, Narkbunnam R. Increased cartilage volume after injection of hyaluronic acid in osteoarthritis knee patients who underwent high tibial osteotomy[J]. Knee Surg Sports Traumatol Arthrosc, 2014, 22(6):1415-1423.
[73] Khalaj N, Abu Osman NA, Mokhtar AH, et al. Effect of Intra-Articular Hyaluronic Injection on Postural Stability and Risk of Fall in Patients with Bilateral Knee Osteoarthritis[J]. Scientific World Journal, 2014, 2014:815184.
[74] Trojian TH, Concoff AL, Joy SM, et al. AMSSM Scientific Statement Concerning Viscosupplementation Injections for Knee Osteoarthritis: Importance for Individual Patient Outcomes[J]. Clin J Sport Med, 2016, 26(1):1-11.
[75] 张志毅,段新旺,古洁若,等.欧洲骨质疏松和骨关节炎临床及经济学协会(ESCEO)和中国骨关节炎领域专家联合发表声明:ESCEO膝骨关节炎治疗规则应同样适用于中国患者[J].中国实用内科杂志,2016,36(9):762-772.
[76] Bannuru RR, Vaysbrot EE, Sullivan MC, et al. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis[J]. Semin Arthritis Rheum, 2014, 43(5):593-599.
[77] Waddell DD, Bricker DC. Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis[J]. J Manag Care Pharm, 2007, 13(2):113-121.
[78] Waddell DD, Joseph B. Delayed Total Knee Replacement with Hylan G-F 20[J]. J Knee Surg, 2016, 29(2):159-168.
[79] Altman R, Lim S, Steen RG, et al. Hyaluronic Acid Injections Are Associated with Delay of Total Knee Replacement Surgery in Patients with Knee Osteoarthritis: Evidence from a Large U.S. Health Claims Database[J]. PLoS One, 2015, 10(12):e0145776.
[80] Shewale AR, Barnes CL, Fischbach LA, et al. Comparison of Low-, Moderate-, and High-Molecular-Weight Hyaluronic Acid Injections in Delaying Time to Knee Surgery[J]. J Arthroplasty, 2017, 32(10):2952-2957.
[81] Anand A, Balduini F, Rogers K. Hyaluronic acid in management of advanced osteoarthritis of the knee: retrospective analysis[J]. J Arthroplasty, 2016, 31(10):2115-2118.
[82] Heybeli N, Doral MN, Atay OA, et al. Intra-articular sodium hyaluronate injections after arthroscopic debridement for osteoarthritis of the knee: a prospective, randomized, controlled study[J]. Acta Orthop Traumatol Turc, 2008, 42(4):221-227.
[83] Li X, Shah A, Franklin P, et al. Arthroscopic debridement of the osteoarthritic knee combined with hyaluronic acid (Orthovisc) treatment[J]. J Orthop Surg Res, 2008, 3(1):43.
[84] Mathies B. Effects of Viscoseal, a synovial fluid substitute, on recovery after arthroscopic partial meniscectomy and joint lavage[J]. Knee Surg Sports Traumatol Arthrosc, 2006, 14(1):32-39.
[85] Hempfling H. Intra-articular hyaluronic acid after knee arthroscopy: a two year study[J]. Knee Surg Sports Traumatol Arthrosc, 2007, 15(5):537-546.
[86] 王维山,董金波,王永明,等.关节镜清理术与关节镜清理术联合玻璃酸钠治疗膝骨关节炎的疗效对比[J].中国内镜杂志,2011,17(2):121-123,127.
[87] Han SH, Park DY, Kim TH. Prognostic factors after intra-articular hyaluronic acid injection in ankle osteoarthritis[J]. Yonsei Med J, 2014, 55(4):1080-1086.
[88] Görmeli G, Karakaplan M, Görmeli CA, et al. Clinical Effects of Platelet-Rich Plasma and Hyaluronic Acid as an Additional Therapy for Talar Osteochondral Lesions Treated with Microfracture Surgery. A Prospective Randomized Clinical Trial[J]. Foot Ankle Int, 2015, 36(8):891-900.
[89] Mei-Dan O, Carmont M, Laver L, et al. Intra-articular injections of hyaluronic acid in osteoarthritis of the subtalar joint: a pilot study[J]. J Foot Ankle Surg, 2013, 52(2):172-176.
[90] Chang KV, Hsiao MY, Chen WS, et al. Effectiveness of intra-articular hyaluronic acid for ankle osteoarthritis treatment: a systematicreview and meta-analysis[J]. Arch Phys Med Rehabil, 2013, 94(5):951-960.
[91] Murphy EP, Curtin M, McGoldrick NP, et al. Prospective Evaluation of Intra-Articular Sodium Hyaluronate Injection in the Ankle[J]. J Foot Ankle Surg, 2017, 56(2):327-331.
[92] Paula Pereira Junior A, Fasolin RP, Sossa FA, et al. Results evaluation of the use of intra-articular sodium hyaluronate in the post-operative knee arthroscopy[J]. Rev Bras Ortop, 2014, 49(1):37-43.
[93] Westrich G, Schaefer S, Walcott-Sapp S, et al. Randomized Prospective Evaluation of Adjuvant Hyaluronic Acid Therapy Administered After Knee Arthroscopy[J]. Am J Orthop (Belle Mead NJ), 2009, 38(12):612-616.
[94] Anand S, Singisetti K, Srikanth KN, et al. Effect of Sodium Hyaluronate on Recovery after Arthroscopic Knee Surgery[J]. J Knee Surg, 2016, 29(6):502-509.
[95] Osti L, Buda M, Buono AD, et al. Clinical evidence in the treatment of rotator cuff tears with hyaluronic acid[J]. Muscles Ligaments Tendons J, 2016, 5(4):270-275.
[96] Moghtaderi A, Sajadiyeh S, Khosrawi S, et al. Effect of subacromial sodium hyaluronate injection on rotator cuff disease. A double-blind placebo-controlled clinical trial[J]. Adv Biomed Res, 2013, 2:89.
[97] Lim TK, Koh KH, Shon MS, et al. Intra-articular injection of hyaluronate versus corticosteroid in adhesive capsulitis[J]. Orthopedics, 2014, 37(10):e860-e865.
[98] Kwon YW, Eisenberg G, Zuckerman JD. Sodium hyaluronate for the treatment of chronic shoulder pain associated with glenohumeral osteoarthritis. a multicenter, randomized, double-blind, placebo-controlled trial[J]. J Shoulder Elbow Surg, 2013, 22(5):584-594.
[99] Park KD, Nam HS, Lee JK, et al. Treatment effects of ultrasound-guided capsular distension with hyaluronic acid in adhesive capsulitis of the shoulder[J]. Arch Phys Med Rehabil, 2013, 94(2):264-270.
[100] Porcellini G, Merolla G, Giordan N, et al. Intra-articular glenohumeral injections of HYADD®4-G for the treatment of painful shoulderosteoarthritis: a prospective multicenter, open-label trial[J]. Joints, 2016, 3(3):116-121.
[101] Marcheggiani Muccioli GM, Wykes P, Hundle B, et al. Effects of a synovial fluid substitute on early recovery after arthroscopic subacromial decompression of the shoulder[J]. Musculoskelet Surg, 2015, 99(2):121-126.
[102] Rivera F. Single intra-articular injection of high molecular weight hyaluronic acid for hip osteoarthritis[J]. J Orthop Traumatol, 2016, 17(1):21-26.
[103] Piccirilli E, Oliva F, Murè MA, et al. Viscosupplementation with intra-articular hyaluronic acid for hip disorders. A systematic review and meta-analysis[J]. Muscles Ligaments Tendons J, 2016, 6(3):293-299.
[104] Abate M, Salini V. Efficacy and safety study on a new compound associating low and high molecular weight hyaluronic acid in the treatment of hip osteoarthritis[J]. Int J Immunopathol Pharmacol, 2017, 30(1):89-93.
[105] Abate M, Scuccimarra T, Vanni D, et al. Femoroacetabular impingement: is hyaluronic acid effective?[J]. Knee Surg Sports Traumatol Arthrosc, 2014, 22(4):889-892.
[106] Lee YK, Lee GY, Lee JW, et al. Intra-Articular Injections in Patients with Femoroacetabular Impingement. a Prospective, Randomized, Double-blind, Cross-over Study[J]. J Korean Med Sci, 2016, 31(11):1822-1827.
[107] Khan W, Khan M, Alradwan H, et al. Utility of Intra-articular Hip Injections for Femoroacetabular Impingement. A Systematic Review[J]. Orthop J Sports Med, 2015, 3(9):2325967115601030.
[108] Shang XL, Tao HY, Chen SY, et al. Clinical and MRI outcomes of HA injection following arthroscopic microfracture for osteochondral lesions of the talus[J]. Knee Surg Sports Traumatol Arthrosc, 2016, 24(4):1243-1249..
[109] Abate M, Schiavone C, Salini V. The use of hyaluronic acid after tendon surgery and in tendinopathies[J]. Biomed Res Int, 2014, 2014:783632.
[110] Frizziero A, Vittadini F, Barazzuol M, et al. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non-calcific rotator cuff tendinopathies: preliminary results[J]. J Sports Med Phys Fitness, 2017, 57(9):1162-1168.
[111] Flores C, Balius R, Álvarez G, et al. Efficacy and Tolerability of Peritendinous Hyaluronic Acid in Patients with Supraspinatus Tendinopathy: a Multicenter, Randomized, Controlled Trial[J]. Sports Med Open, 2017, 3(1):22.
[112] Lynen N, De Vroey T, Spiegel I, et al. Comparison of Peritendinous Hyaluronan Injections Versus Extracorporeal Shock Wave Therapy in the Treatment of Painful Achilles' Tendinopathy: A Randomized Clinical Efficacy and Safety Study[J]. Arch Phys Med Rehabil, 2017, 98(1):64-71.
[113] Liu DH, Tsai MW, Lin SH, et al. Ultrasound-Guided Hyaluronic Acid Injections for Trigger Finger: A Double-Blinded, Randomized Controlled Trial[J]. Arch Phys Med Rehabil, 2015, 96(12):2120-2127.
[114] Lussier A, Cividino AA, McFarlane CA, et al. Viscosupplementation with hylan for the treatment of osteoarthritis: Findings from clinical practice in Canada[J]. J Rheumatol, 1996, 23(9):1579-1585.
[115] Conrozier T, Patarin J, Mathieu P, et al. Steroids, lidocain and ioxaglic acid modify the viscosity of hyaluronic acid: in vitro study and clinical implications[J]. Springerplus, 2016, 5:170.
引用本文:中国医师协会骨科医师分会运动医学专业委员会.玻璃酸钠在骨科和运动医学相关疾病中的应用专家共识(2017年修订版)[J].中国医学前沿杂志(电子版),2017,9(11):1-8.


 京公网安备11010502051256号
京公网安备11010502051256号